Introduction
Early progression of FL after first line treatment is associated with poor outcomes. Disease progression within 24 months of initial therapy (POD24) is well-established adverse prognostic factor, while maintaining a complete remission (CR) at 30 months after initiation of induction therapy is considered a good surrogate for a prolonged progression-free survival (PFS). Nevertheless, treatment strategies in the second-line setting for these early relapsing patients are not clearly established, being their management heterogeneous across centers.
The aim of our study was to analyze treatment patterns in this setting and to compare outcomes for patients who received chemotherapy (CT)-based regimens and immunotherapy (IT) approaches.
Methods
We conducted a retrospective review of patients diagnosed with grade 1-3a FL treated with upfront (CT) that progressed within 30 months of initial therapy (POD30) in 9 centers from January 2015 to June 2023. Patients who received single agent monoclonal antibodies or radiotherapy as first line treatment, and patients with FL grade 3b or histological transformation were excluded from the analysis. We defined 2 groups of patients according to the second-line regimen: the CT group, comprised by patients who received bendamustine-based regimens or other CT combinations, and the IT group that included patients who received monoclonal antibodies with lenalidomide and/or CD20xCD3 bispecific antibodies. Outcomes of interest were overall and complete response rate (ORR/CRR), PFS and overall survival (OS).
Results
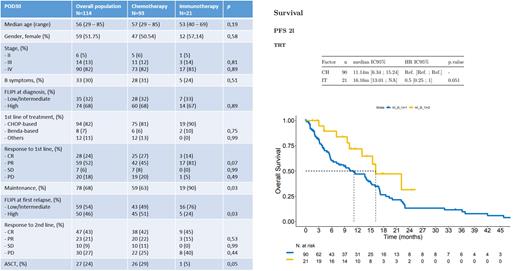
Among 114 POD30 FL patients, 93 received CT and 21 received IT as second line treatment. Median age for the overall cohort was 56 years, with no differences according to salvage therapy (57 vs 53 years, p=0.19). Fifty-nine patients (52%) were female and 104 (94%) presented an advanced stage disease. Most of the patients in both groups had a high-risk FL at diagnosis (68% vs 67%, p=0.89) and at the time of first relapse (51% vs 24%, p=0.03), respectively.
Distribution of first line treatment was well balanced between both groups. CHOP-based regimens were administered in 81% patients from the CT group and 90% in the IT group while bendamustine-based regimens were administered in 6% patients from the CT group and 10% in the IT group. In terms of best response to frontline therapy, 28 patients (25%) attained a CR, 59 patients (52%) a partial response (PR), 7 patients (6%) a stable disease (SD) and 20 patients (18%) a progressive disease (PD). Maintenance treatment was administered in 78 patients (68%), 59 patients (63%) from the CT group and 19 patients (90%) from the IT group ( p=0.03).
Second-line treatment in the CT group included bendamustine-based regimens (n=40, 43%), ESHAP (n=31, 33%), gemcitabine-based (n=7, 8%), and other regimens (n=15, 16%), while the IT cohort comprised patients treated with lenalidomide in combination with monoclonal antibodies (n=5, 24%) and bispecific antibodies (n=16, 76%). For the full set of patients, best response included CR in 47 patients (43%), PR in 23 patients (21%), SD in 10 patients (9%) and PD in 30 patients (18%). For the CT and IT groups, there were no significant differences in terms of ORR (64% vs 60%, p=0.66) or CRR (42% vs 45%, p=0.87), respectively. Autologous stem cell transplantation (ASCT) was performed as a consolidative strategy in 27 patients (24%) from the overall cohort (29% for the CT cohort and 5% for the IT, p=0.05).
Median follow up was 39.2 from diagnosis and 15.4 months from second-line treatment. Median PFS for patients receiving CT as second line of treatment was 11 months (95% CI: 6.3 - 15.2), compared to 16 months (95% CI: 13.01 - NA) for patients receiving IT (HR: 0.5, CI 95% 0.25-1; p=0.05). Finally, median OS for patients receiving CT as salvage therapy was 45.6 months (95% CI: 24.15% - NA), while median OS was not reached for patients receiving IT (HR: 0.19, CI 95% 0.03-1.4; p=0.1).
Conclusions
Treatment approaches for early relapsing FL are heterogeneous across centers, with many patients receiving chemotherapy as salvage therapy. Nevertheless, in our study a trend to a prolonged PFS and OS is observed in the group of patients receiving immunotherapy as salvage treatment. Novel agents such as lenalidomide and bispecific antibodies alone or in combination seem to improve the outcome in this population of FL with poor prognosis.
Disclosures
Lopez Garcia:Beigene: Consultancy; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau. Cordoba:European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; Fundacion Jimenez Diaz University Hospital: Current Employment. Cabirta Touzón:AstraZeneca: Honoraria; Janssen: Honoraria; AbbVie: Other; BeiGene: Honoraria. Marin Niebla:Lilly: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Kiowa Kirin: Consultancy; Takeda: Consultancy, Honoraria; AstraZeneca: Consultancy; Roche: Consultancy; Janssen: Consultancy, Honoraria. Iacoboni:AstraZeneca: Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Honoraria; MSD: Honoraria; Novartis: Consultancy, Honoraria; Abbvie: Honoraria; Autolus: Consultancy; Miltenyi: Consultancy, Honoraria. Bosch:Abbvie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Karyospharm: Other; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Costa:Genmab: Consultancy, Honoraria; Astrazeneca: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal